
- Home
-
About UsIntroduction History Quality Qualification
- Products
CHO Stably Transfected Series Transiently Transfected Series Other product series- Services
IntroductionFounded in 2018, CelluPro is a modern high-tech enterprise specializing in the development, production (OEM) and sales of high quality serum-free medium for mammalian cells. We have decades of experience in R&D and industrialization in mammalian cell expression system.
With excellent product performance, CelluPro's medium products are widely used and recognized in recombinant cell line development, cell culture process development and commercial production. We are the first Chinese medium company that supports listing of Chinese first-in-class new drug and ADC new drug, making our medium more competitive than similar products in the market.
Under the philosophy of "professional, credible and innovative", CelluPro develops on innovation and aims to offer strong support for commercialization in biopharmaceutical industry.
Under people-oriented management, we pursue value that benefits both employees and the company, and focus on our customer's need. We believe that win-win cooperation can be realized with customers under the guidance of advanced company philosophy and technology!
History2023.12Successfully declared as a high-tech enterprise and received special funds from the government
2022.5Declared as high-tech SME
2021.12Received national dedicated funds to guide local scientific and technological development
2021.11Certified by Environmental Management System ISO45001:2018 and Occupational Health and Safety System ISO14001:2015
2021.9Certified by Quality Management System ISO9001:2015 and ISO13485:2016
2021.5Supported listing of Chinese first self-developed ADC new drug
2021.3Supported listing of RemeGen's first-in-class new drug RC18
2020.12Powder medium production line with an annual output of one million liters went into services
2019.4Business expanded overseas
2018.06.27CelluPro was founded and established long-term strategic supply partnership of medium with RemeGen
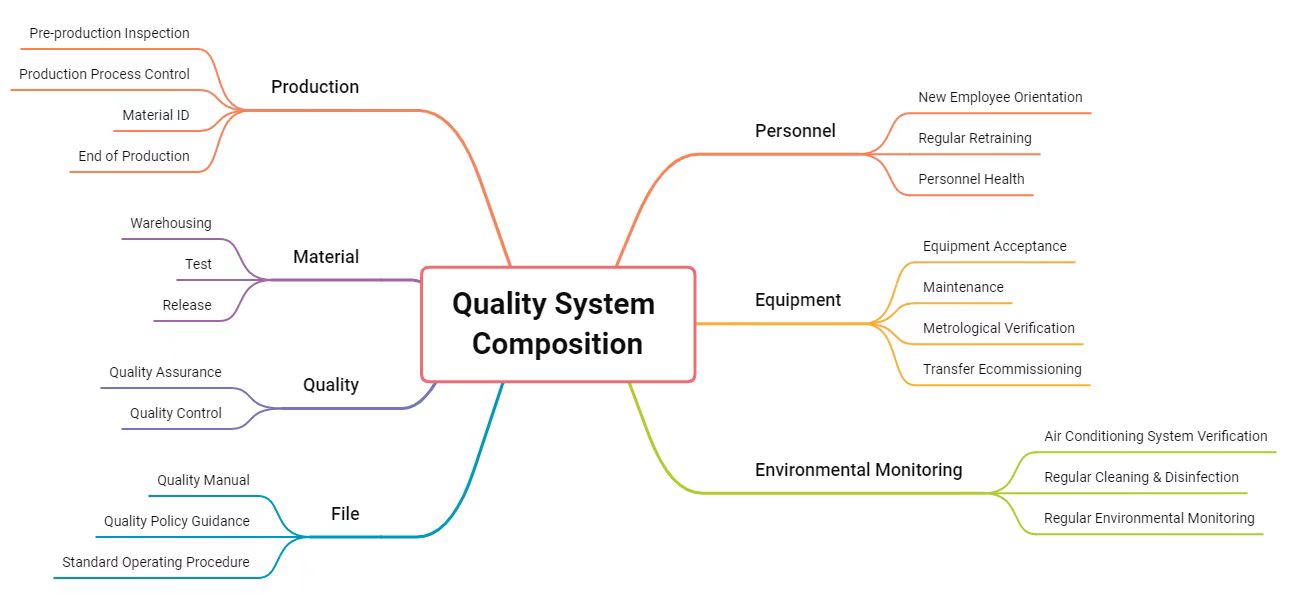
QualityCelluPro attaches great importance to the construction of quality management system. With a quality policy prioritizing quality, credit, management and service, we have a complete quality system that monitors the whole production process from the perspectives of human, equipment, material, method, and environment, to provide customers with high quality products and services. We are certified by ISO9001, ISO13485, ISO14001 and ISO45001.
- Contact Us
- Address:60 Beijing Middle Road, Economic & Technological Development Area, Yantai Municipality, Shandong Province, China
- CONTACT
- HR:Tel.:+86-535-3573399,Email:hr@cellupro.cn
- Sales department:Tel:+86-535-3573396,Email:sales@cellupro.cn
- Technical support:Tel:+86-535-3573480,Email:support@cellupro.cn
 WeChat
Copyright ©2022 CelluPro,Ltd. All rights reserved.
WeChat
Copyright ©2022 CelluPro,Ltd. All rights reserved. - Products


